under what environmental conditions would a plant use its stored starch to power its metabolism
Starch Metabolism: Enzymatic Machinery and Regulation
Plants accumulate starch as both a transient and long-term saccharide reserve. As a result, starch metabolism must be adjusted to provide ample carbon supply in response to many physiological demands mainly related to nocturnal, stress, and formation events (Stitt and Zeeman, 2012; Lloyd and Kossmann, 2015; Hedhly et al., 2016; Zanella et al., 2016; Pirone et al., 2017; Thalmann and Santelia, 2017). Coordinated metabolic flux amidst starch biosynthetic enzymes can also permit correct structuring of the starch granule irrespectively of carbon flow (Blennow and Svensson, 2010; Glaring et al., 2012; Pfister and Zeeman, 2016; Botticella et al., 2018).
The reactions of starch metabolism are catalyzed by a series of enzymes (Figure i), mainly regulated to account for transitory starch biosynthesis during active photosynthesis and mobilization at night (MacNeill et al., 2017). These reactions can be functionally classified equally constructed or degradative reactions. However, these should not be considered every bit strictly culling reactions every bit transitory starch degradation is suggested to occur simultaneously with starch synthesis under long twenty-four hours conditions (Fernandez et al., 2017).
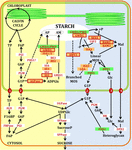
FIGURE ane. Pathways of starch synthesis (yellow) and degradation (blue) in Arabidopsis leaves. Orange boxes with crimson outlines represent well confirmed redox regulated enzymes. Orange boxes with no outlines represent suggested redox regulated enzymes. Green boxes represent redox tolerant enzymes. AM, amylose; AP, amylopectin; TP, triosephosphates; F6P, fructose-6-phosphate; G6P, glucose-6-phosphate; G1P, glucose-1-phosphate; ADPGlc, ADP-glucose; MOS, maltooligosaccharides; Mal, maltose; Glc, glucose; F16BP, fructose-1,6-bisphosphate; UDPGlc, UDP-glucose; SucroseP, sucrose-phosphate; ALD, aldolase; FBPase, fructose-one,6-bisphosphatase; PGI, phosphoglucose isomerase; PGM, phosphoglucomutase; AGPase, ADP-glucose pyrophosphorylase; SS, soluble starch synthase isoforms; GBSS, granule-leap starch synthase; Exist, branching enzyme; ISA, isoamylase; LDA, limit dextrinase; PHS, α-glucan phosphorylase; GWD1, glucan water dikinase; PWD, phosphoglucan, water dikinase; SEX4 and LSF2, phosphoglucan phosphatases; BAM, β-amylase; AMY, α-amylase; DPE, disproportionating enzyme; FBPase, fructose-1,6-bisphosphatase; UGPase, UDP-glucose pyrophosphorylase; SPSase, sucrose-phosphate synthase; SPPase, sucrose phosphate phosphatase; HK, hexokinase. Transporters are shown as cherry-red filled circles: (one) triose-phosphate/phosphate translocator (TPT); (ii), G1P translocator; (3) plastidial glucose transporter (pGLT); (4) maltose transporter (MEX1). Dashed arrows represent pocket-sized or possible routes. Figure inspired from Stitt and Zeeman (2012).
To permit tight advice between energy demands at a multitude of provisional situations, starch metabolism is regulated at several levels (Kötting et al., 2010; Stitt and Zeeman, 2012; MacNeill et al., 2017). The rate of both starch biosynthesis every bit well every bit starch degradation correlates with the anticipated length of the night directly or reversibly (Smith and Stitt, 2007), providing evidence of a tight circadian command (Graf et al., 2010). Moreover, simultaneously monitoring photosynthetic rate, soil minerals availability, various abiotic and biotic stress factors requires precise mechanisms adjusting the rate of transient starch turnover in response to these stimuli (Thalmann and Santelia, 2017) and both transcriptional and post-translational levels are important (Geigenberger, 2011; Stitt and Zeeman, 2012; Geigenberger and Fernie, 2014; Santelia et al., 2015; Mahlow et al., 2016; MacNeill et al., 2017).
Regulation at the transcriptional level provides a mid- to long-term aligning of starch turnover (Stitt and Zeeman, 2012) while post-translational modifications are currently accustomed to be the primary way by which the diel periodic activeness of enzymes of transient starch metabolism are regulated (Kötting et al., 2010). The latter include diverse allosteric mechanisms, phosphorylation dependent poly peptide–protein complexation and redox mediated cysteine modification (Table i). Information that emerged over the concluding two decades advise that the redox state of the cell plays an of import analogous part in cellular homeostasis by regulation of starch metabolism. Such thiol-based redox mechanisms are more pronounced in eukaryotes than prokaryotes and in also more important in photosynthetic organisms as compared to heterotrophic ones. This suggests an importance of tight link between energy harvest and downstream metabolism in circuitous autotrophic organisms.

Table one. Redox regulated starch metabolic enzymes.
Redox-Active Enzymes in Starch Metabolism
A large number of plastidial enzymes involved in carbohydrate metabolism are demonstrated to exist redox-sensitive as delineated in Effigy 1. These data identify enzymes both in the biosynthetic and the degradation pathways, equally mainly achieved by using well-defined in vitro systems (Table 1) and are every bit such only indicative for a cellular function. However, such mechanistic studies take given important information to identify potential target enzymes, cross-links between photosynthesis and stress-related metabolic steps.
Many of the enzymes demonstrated to be redox sensitive has been thoroughly studied in vitro and shown to (i) modulate enzyme activity, (ii) depend on physiological reductants, and (iii) depend on specific poly peptide cysteine(s). Based on these criteria, enzymes of starch metabolism similar AGPase, SS1, GWD1, SEX4, BAM1, and AMY3 tin be considered redox-regulated. If some of these criteria are non fulfilled, nosotros denote enzymes, including SS3, BE2, ISA1/2/3, BAM3, and LDA, every bit suggested to be redox regulated, implying that farther studies in vitro and in vivo, should exist performed to confirm the regulation.
The principal identified mechanism involves cystin reversible exchange mediated by thioredoxins (Trxs) having different redox potentials (Yoshida and Hisabori, 2017) and NADP-dependent thioredoxin reductase C (NTRC) leading to conformational change in the target enzyme. Both Trx and NTRC are efficient redox transmitters and for instance AGPase and BAM1 are starch-metabolic enzymes known to be reduced by Trx f, m and NTRC (nigh) equally well (Valerio et al., 2011; Thormählen et al., 2013). Typically, target enzymes lose catalytic activity upon oxidation (Couturier et al., 2013) and increased analogousness to starch, as for the starch phosphorylator GWD1 (Mikkelsen et al., 2005) and SS1 (Skryhan et al., 2015; Tabular array 1).
Emerging Evidence of Redox Regulation of Transitory Starch Metabolism In Vivo
In few cases (AGPase, GWD1), redox regulation was shown also in vivo to exist influenced by external weather such every bit illumination or sugar supply (Hendriks et al., 2003; Kolbe et al., 2005; Hädrich et al., 2012; Skeffington et al., 2014). In most other cases, however, in vivo data are completely lacking. This section deals with redox regulation of starch metabolism in vivo.
In leaves, AGPase is highly reduced in the light but is increasingly reduced as well in the dark when leaves are supplied with sucrose (Hendriks et al., 2003). In vitro, AGPase is reduced by thioredoxins (mainly f and g) and past NTRC (Ballicora et al., 2000; Thormählen et al., 2013) and in this way it becomes more than sensitive to 3-phosphoglycerate activation (Fu et al., 1998; Ballicora et al., 2000). AGPase reduction, as directly measured in establish extracts, is thus taken as a proxy of its actual activity in vivo. Consistently, atmospheric condition that pb to AGPase reduction often stimulate as well starch aggregating and vice versa, supporting the view that AGPase, in leaves, is reduced/activated in the light by thioredoxins photoreduced by photosystem I (PS-I) via ferredoxin/thioredoxin reductase (FTR), and reduced/activated by sucrose through a different pathway, operative also in the dark, that involves trehalose-6P and NTRC (Kolbe et al., 2005). This view could be too simplistic, only is essentially accepted.
To which extent redox regulation is important for starch metabolism cannot, however, be easily assessed from this kind of experiments. Arguably, reverse genetics provides the more than straightforward approaches to address this question. Mutant plants in which the redox regulated cysteine(s) of a redox regulated enzyme are substituted by redox-inactive amino acid residues like serine plant in principle the best found material for studying the relevance of redox regulation in a physiological context. To our best knowledge, this approach has been applied merely in ii cases in starch metabolism, to AGPase (Hädrich et al., 2012) and GWD1 (Skeffington et al., 2014).
AGPase has the analytical reward that only a single cysteine (Cys-81 in Arabidopsis) of its small subunit APS1 is both necessary and sufficient for its redox regulation, which consists in the formation of a disulfide span betwixt 2 APS1 subunits of the tetrameric enzyme. Mutants with a serine substituting Cys-81 cannot form the disulfide bridge and are permanently redox-activated (Fu et al., 1998; Hädrich et al., 2012). Arabidopsis lines expressing such a mutagenized and permanently agile AGPase in a AGPase-knockout background, contain more leaf starch when grown in long-days photoperiods. Interestingly, this was suggested not to be an effect of faster starch synthesis in the light, merely slower starch degradation during the night (Hädrich et al., 2012). Hence, a function for AGPase redox regulation in starch turnover was demonstrated, but the metabolic mechanisms are far from clear. Further observations revealed that the mutagenized AGPase, in vivo, was degraded faster than the wild type enzyme (Hädrich et al., 2012), suggesting that its physiological turnover was influenced by the redox state of the protein. In wild type plants, oxidized AGPase might be somehow protected from the rapid deposition suffered by mutagenized AGPase, which mimics the reduced form. This hypothesis suggests an boosted role of redox regulation in poly peptide turnover (van Wijk, 2015). Such a mechanism is reminiscent of phosphoribulokinase (PRK) that is rapidly degraded in plants in which the three genes of CP12, a protein that redox regulates PRK and GAPDH by assembling a supramolecular circuitous (Marri et al., 2008, 2009), accept been knocked out (López-Calcagno et al., 2017). It has also been shown that oxidized GWD1 from potato (Mikkelsen et al., 2005) and recombinant SS1 from Arabidopsis thaliana (Skryhan et al., 2015) have the enhanced affinity for the surface of starch granules. A possible caption of this phenomenon is that such mechanism is necessary for the enzymes protection under oxidative stress.
GWD1 is the main starch phosphorylator in the establish cell (Blennow and Engelsen, 2010). In vitro, GWD1 is completely inactive when disulfide oxidized in the CFATC motif (Mikkelsen et al., 2005). Oxidized GWD tends to bind starch granules in the dark, but a portion of GWD1 remains reduced and soluble in the stroma (Mikkelsen et al., 2005). Interestingly, the redox potential of GWD1 regulatory disulfide (East°' −250 mV) is much less negative than that of thioredoxin f (E°'−290 mV), suggesting that GWD1 would be reduced in vivo under normal weather (Yoshida et al., 2014). Nonetheless, granule-bound oxidized GWD1 might be less easily reduced by Trx f. The relevance of GWD1 for diurnal starch regulation has been questioned by reverse genetic studies. It turns out that Arabidopsis gwd mutants have a stiff starch excess phenotype but recover a quasi-normal starch turnover if complemented with, either the wild type, redox-regulated, course or the redox insensitive, mutagenized, GWD1 form (Skeffington et al., 2014). Hence, the physiological role of GWD1 is unclear and it remains to be tested whether the starch granule affinity of the oxidized form (Mikkelsen et al., 2005) can take a protective role, peradventure during astringent stress weather as mentioned above.
Another possibility to test redox regulation in vivo is to study mutants of regulatory proteins, specially Trxs, NTRC. All the same, since both Trxs and NTRC take multiple targets (Cejudo et al., 2012; Pérez-Pérez et al., 2017), results obtained by this approach should be interpreted with caution considering of pleiotropic effects. The Arabidopsis genome codes for 5 classes of plastidial Trxs (f, m, x, y, z), each including one (x, z) or more than isoforms (f1, f2; m1, m2, m3, m4; y1, y2) (Meyer et al., 2006). Trxs m1, m2, m4, and f1 are more abundant and, amongst the 10 isoforms overall, constitute about 90% of the protein content (Okegawa and Motohashi, 2015). In vitro, different Trxs have different redox potentials and are reduced by FTR with different efficiency (Yoshida and Hisabori, 2017). In vivo, these Trxs are more reduced in the light than in the night, with differences amid isoforms (Yoshida et al., 2014).
Single knockout mutants for Trx f1 in Arabidopsis, did not show any visible defect in growth or photosynthetic performance, just accumulated less leafage starch and showed a lower activation state of AGPase (Thormählen et al., 2013). Tobacco plants that overexpress Trx f conversely accumulate large amounts of starch in chloroplasts, and make more leaf biomass, although the redox state of AGPase was not affected (Sanz-Barrio et al., 2013). Intriguingly, NTRC overexpression besides has a positive outcome on growth of Arabidopsis plants and accumulated more starch in leaves (Toivola et al., 2013; Nikkanen et al., 2016). NTRC has been demonstrated to promote starch aggregating in response to light or external sucrose treatment via redox-dependent AGPase activation (Michalska et al., 2009). Like to trx f1 plants, double trxf1-f2 mutants also accumulated less starch at the cease of the twenty-four hours but, dissimilar from trx f1 plants, also showed more than general phenotypic defects including growth retardation in curt-day photoperiods and lower photosynthetic send rates (Naranjo et al., 2016; Ojeda et al., 2017). Depression levels of starch were also institute in ntrc and in trx 10 single mutants (Ojeda et al., 2017), although Arabidopsis AGPase is efficiently activated past NTRC but not as much by Trx x (Thormählen et al., 2013). Overall, a scenario seems to emerge in which the level of redox regulatory proteins like Trx f, Trx x, and NTRC correlate with the amount of transitory starch and, in some cases, with growth. Combinations of double and triple mutants similar ntrc-trx x and ntrc-trxf1-f2 confirm the trend since plants impaired in these redox regulatory proteins tend to shop less starch in leaves (Ojeda et al., 2017). Even so, additional effects such every bit astringent growth inhibition, perturbed low-cal acclimation, and impairment of Calvin–Benson cycle activity prevents a elementary estimation of these results.
The complexity of redox regulatory systems was nicely demonstrated by the suppressed growth phenotype of ntrc mutants by simultaneous knocking out ii ii-Cys peroxiredoxins (2CP, thiol peroxidases involved in antioxidant defense and redox signaling, Pérez-Ruiz et al., 2017). It was proposed that in chloroplasts of wild blazon plants NTRC is especially involved in reducing 2CP and thus H2O2, while typical Trxs are reduced past FTR to go along the Calvin–Benson bicycle activated in the lite. The growth defect of ntrc mutant is explained by the capacity of 2CP, in the absenteeism of NTRC, to drain electrons from the Trx puddle, thereby causing an indirect downregulation of the Calvin–Benson wheel. Abolishing the electron withdrawal by additional knocking out the 2CPs restores the capacity of FTR-reduced Trxs to activate the Calvin–Benson cycle and thereby growth (Pérez-Ruiz et al., 2017). Clearly, phenotypes of knockout mutants of redox regulatory proteins (like ntrc) must be interpreted with care.
Transitory Starch Re-Cycling: the Current Argue on Simultaneous Biosynthesis and Degradation of Starch in the Calorie-free
In the last two decades the pathway of transitory starch breakdown has been deeply detailed (Yu et al., 2001; Ritte et al., 2002; Niittylä et al., 2004; Fulton et al., 2008; Kötting et al., 2009). The widely accepted model describes a night-active pathway for starch deposition to balance the lack of triose phosphates from the Calvin–Benson cycle. Accordingly, starch behaves as a carbon buffer to fuel plant metabolism and growth when photosynthesis is inactive (Zeeman et al., 2010; Stitt and Zeeman, 2012). However, there is support for the existence of starch deposition in illuminated leaves both in the absence (Stitt and Heldt, 1981; Baslam et al., 2017; Fernandez et al., 2017) and in the presence (Valerio et al., 2011; Feike et al., 2016; Thalmann et al., 2016; Zanella et al., 2016) of stress.
There is a general agreement that stresses ranging from increased photorespiratory charge per unit to more severe osmotic stress, trigger foliage starch degradation in low-cal (Lu et al., 2005; Weise et al., 2006; Valerio et al., 2011; Prasch et al., 2015; Thalmann et al., 2016; Zanella et al., 2016). In relation to redox regulation, information technology is worth mentioning that degradative enzymes can also be reductively activated. At first sight, reductive activation of enzymes involved in starch degradation is counterintuitive since such degradation would interfere with active starch accumulation during the day and loss of activity during the night-fourth dimension. Nevertheless, starch deposition has actually been shown to take place simultaneously with starch synthesis nether long day atmospheric condition (Fernandez et al., 2017). Starch degradation during the day tin can also play a physiological function under certain stress conditions as demonstrated for AtBAM1 beingness active upon osmotic stress (Valerio et al., 2011) and AtAMY3 showing increased expression later cold daze. Additionally, starch accumulation was elevated in mutants lacking AMY3 (Seung et al., 2013). Another option is a spatial separation of starch degradation which was demonstrated for guard cells where starch degradation by the BAM1 sustains stomata opening (Valerio et al., 2011; Santelia et al., 2015). Power of some redox-sensitive targets to exist activated by NTRC, which takes the reducing power from the calorie-free-independent oxidative pentose phosphate pathway, tin provide a reductive activation of starch degrading enzymes in the dark.
To what extent diurnal starch degradation contributes to starch turnover in the light is however debated. Two very recent studies have demonstrated agile diurnal starch degradation in plants exposed to continuous low-cal (Baslam et al., 2017; Fernandez et al., 2017). Ane study (Fernandez et al., 2017) proposes that diurnal starch degradation only occurs belatedly in the solar day (over fourteen h from dawn) post-obit the classical pathway illustrated in Figure 1. A second model (Baslam et al., 2017) proposes extensive starch degradation in the light based on a carbon cycle around ADP-glucose (Baroja-Fernández et al., 2004; Baslam et al., 2017). Appropriately, consign of triose phosphate to the cytosol would result in the product of ADP-glucose through the action of sucrose synthase (Baroja-Fernández et al., 2004; Baslam et al., 2017) that could enter the chloroplast (Pozueta-Romero et al., 1991), be converted into starch, and be degraded to sustain found growth even in the light (Baslam et al., 2017). Although intriguing, the main obstacle to this model is that the role of chloroplastic AGPase in the synthesis of starch would exist marginal and the strong defective phenotype of the AGPase mutant hard to explain (Lin et al., 1988a,b; Wang et al., 1997, 1998).
Future Directions
Although meaning advances in the understanding of redox regulation of starch metabolism accept been fabricated, many questions even so remain open. Specially, much inquiry has been conducted on the redox response of chloroplastic enzymes since transitory starch is a major product of leaf photosynthesis through the Calvin–Benson cycle suggested to be strictly redox-regulated (Michelet et al., 2013). Transitory starch metabolism changes dramatically nether light or dark weather condition, and this behavior may link diel starch metabolism to the light-dependent redox state of chloroplast thioredoxins in vivo (Yoshida et al., 2014). Emerging prove supports the view that starch accumulation in illuminated leaves is positively correlated with the reduced state of the starch biosynthetic enzyme AGPase and, in general, with the capacity of the redox regulatory machinery (see section "Emerging Show of Redox Regulation of Transitory Starch Metabolism in vivo"). However, evidence for the physiological relevance of the redox sensitivity of many starch metabolic enzymes is notwithstanding incomplete and stronger biochemical evidence is required.
In vivo studies have clearly demonstrated the relevance of redox regulation for starch metabolism through reverse genetic approaches on redox regulatory proteins (trx f1, Thormählen et al., 2013; trx f1-f2, Naranjo et al., 2016; ntrc and trx ten; Ojeda et al., 2017). Although the analysis of knock out mutants has greatly contributed to the discovery of starch synthesis and degradation pathways, this approach is limited since thioredoxins and NTRC have several targets which complicates the interpretation of data.
Mutating specific cysteines responsible for the redox regulation of metabolic enzymes appears every bit a more promising approach to directly deduce the mechanisms of regulation (Hädrich et al., 2012; Skeffington et al., 2014; Skryhan et al., 2015). The introduction of genome editing opens an exciting scenario since this method allows direct modification of genes of interest, fugitive boosted genetic variations. Nevertheless, any effort to precisely modify a Deoxyribonucleic acid coding sequence in vivo will require deeper biochemical in vitro knowledge of the structure and behavior of the coded poly peptide.
Hence, combinatorial approaches would exist required shed new calorie-free on the importance of redox regulation in starch metabolism. Such information is urgently required considering that starch is central component of food, feed, and time to come materials like bioplastics. Efficient agro-production to feed the doubling globe population in 2050 and to solve primal ecology issues like plastics pollution requires stress-tolerant and robust starch crops. Controlling redox modulation of starch crops is a central point for maximizing crop efficiency in a future changing climate.
Writer Contributions
All authors contributed equally to the content of this paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of whatsoever commercial or financial relationships that could be construed as a potential conflict of involvement.
References
Ballicora, Thou. A., Frueauf, J. B., Fu, Y., Schürmann, P., and Preiss, J. (2000). Activation of the irish potato tuber ADP-glucose pyrophosphorylase past thioredoxin. J. Biol. Chem. 275, 1315–1320. doi: x.1074/jbc.275.ii.1315
CrossRef Full Text | Google Scholar
Baroja-Fernández, E., Muñoz, F. J., Zandueta-Criado, A., Morán-Zorzano, M. T., Viale, A. 1000., Alonso-Casajús, Northward., et al. (2004). Most of ADP⋅glucose linked to starch biosynthesis occurs exterior the chloroplast in source leaves. Proc. Natl. Acad. Sci. U.Due south.A. 101, 13080–13085. doi: x.1073/pnas.0402883101
PubMed Abstract | CrossRef Total Text | Google Scholar
Baslam, M., Baroja-Fernández, Eastward., Ricarte-Bermejo, A., Sánchez-López,ÁM., Aranjuelo, I., Bahaji, A., et al. (2017). Genetic and isotope ratio mass spectrometric evidence for the occurrence of starch degradation and cycling in illuminated Arabidopsis leaves. PLoS One 12:e0171245. doi: 10.1371/journal.pone.0171245
PubMed Abstract | CrossRef Total Text | Google Scholar
Blennow, A., and Engelsen, S. B. (2010). Helix-breaking news: fighting crystalline starch energy deposits in the cell. Trends Constitute Sci. 15, 236–240. doi: ten.1016/j.tplants.2010.01.009
PubMed Abstract | CrossRef Full Text | Google Scholar
Blennow, A., and Svensson, B. (2010). Dynamics of starch granule biogenesis the part of redox-regulated enzymes and low-analogousness saccharide-binding modules. Biocatal. Biotransformation 28, iii–9. doi: 10.3109/10242420903408211
CrossRef Full Text | Google Scholar
Botticella, E., Sestili, F., Sparla, F., Moscatello, S., Marri, Fifty., Cuesta-Seijo, J. A., et al. (2018). Combining mutations at genes encoding key enzymes involved in starch synthesis affects the amylose content, saccharide allocation and hardness in the wheat grain. Plant Biotechnol. J. doi: 10.1111/pbi.12908 [Epub ahead of impress].
PubMed Abstract | CrossRef Full Text | Google Scholar
Cejudo, F. J., Ferrández, J., Cano, B., Puerto-Galán, 50., and Republic of guinea, M. (2012). The role of the NADPH thioredoxin reductase C-2-Cys peroxiredoxin system in plastid redox regulation and signalling. FEBS Lett. 586, 2974–2980. doi: 10.1016/j.febslet.2012.07.003
PubMed Abstruse | CrossRef Full Text | Google Scholar
Feike, D., Seung, D., Graf, A., Bischof, Southward., Ellick, T., Coiro, M., et al. (2016). The starch granule-associated protein Early on STARVATION1 (ESV1) is required for the control of starch degradation in Arabidopsis thaliana leaves. Found Cell 28, 1472–1489. doi: 10.1105/tpc.16.00011
PubMed Abstract | CrossRef Full Text | Google Scholar
Fernandez, O., Ishihara, H., George, M. K., Mengin, V., Flis, A., Sumner, D., et al. (2017). Leaf starch turnover occurs in long days and in falling calorie-free at the end of the day. Plant Physiol. 174, 2199–2212. doi: 10.1104/pp.17.00601
PubMed Abstract | CrossRef Full Text | Google Scholar
Fu, Y., Ballicora, M. A., Leykam, J. F., and Preiss, J. (1998). Mechanism of reductive activation of potato tuber ADP-glucose pyrophosphorylase. J. Biol. Chem. 273, 25045–25052. doi: x.1074/jbc.273.39.25045
PubMed Abstract | CrossRef Full Text | Google Scholar
Fulton, D. C., Stettler, Grand., Mettler, T., Vaughan, C. One thousand., Li, J., Francisco, P., et al. (2008). β-AMYLASE4, a noncatalytic poly peptide required for starch breakdown, acts upstream of iii active β-amylases in Arabidopsis chloroplasts. Plant Cell 20, 1040–1058. doi: x.1105/tpc.107.056507
PubMed Abstract | CrossRef Full Text | Google Scholar
Glaring, M. A., Skryhan, Chiliad., Kötting, O., Zeeman, S. C., and Blennow, A. (2012). Comprehensive survey of redox sensitive starch metabolising enzymes in Arabidopsis thaliana. Plant Physiol. Biochem. 58, 89–97. doi: x.1016/j.plaphy.2012.06.017
PubMed Abstruse | CrossRef Full Text | Google Scholar
Graf, A., Schlereth, A., Stitt, M., and Smith, A. Yard. (2010). Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc. Natl. Acad. Sci. 107, 9458–9463. doi: 10.1073/pnas.0914299107
PubMed Abstract | CrossRef Total Text | Google Scholar
Hädrich, N., Hendriks, J. H. 1000., Kötting, O., Arrivault, S., Feil, R., Zeeman, S. C., et al. (2012). Mutagenesis of cysteine 81 prevents dimerization of the APS1 subunit of ADP-glucose pyrophosphorylase and alters diurnal starch turnover in Arabidopsis thaliana leaves. Plant J. lxx, 231–242. doi: 10.1111/j.1365-313X.2011.04860.x
PubMed Abstruse | CrossRef Full Text | Google Scholar
Hedhly, A., Vogler, H., Schmid, G. W., Pazmino, D., Gagliardini, V., Santelia, D., et al. (2016). Starch turnover and metabolism during blossom and early embryo evolution. Plant Physiol. 172, 2388–2402. doi: 10.1104/pp.16.00916
PubMed Abstract | CrossRef Total Text | Google Scholar
Hendriks, J. H. Chiliad., Kolbe, A., Gibon, Y., Stitt, M., and Geigenberger, P. (2003). ADP-Glucose pyrophosphorylase is activated by posttranslational redox-modification in response to lite and to sugars in leaves of arabidopsis and other constitute species. Plant Physiol. 133, 838–849. doi: x.1104/pp.103.024513
PubMed Abstract | CrossRef Full Text | Google Scholar
Kolbe, A., Tiessen, A., Schluepmann, H., Paul, M., Ulrich, S., and Geigenberger, P. (2005). Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc. Natl. Acad. Sci. United statesA. 102, 11118–11123. doi: 10.1073/pnas.0503410102
PubMed Abstract | CrossRef Full Text | Google Scholar
Kötting, O., Kossmann, J., Zeeman, South. C., and Lloyd, J. R. (2010). Regulation of starch metabolism: the age of enlightenment? Curr. Opin. Plant Biol. xiii, 321–329. doi: x.1016/j.pbi.2010.01.003
PubMed Abstract | CrossRef Full Text | Google Scholar
Kötting, O., Santelia, D., Edner, C., Eicke, South., Marthaler, T., Gentry, M. S., et al. (2009). STARCH-EXCESS4 is a laforin-similar phosphoglucan phosphatase required for starch deposition in Arabidopsis thaliana. Plant Jail cell 21, 334–346. doi: 10.1105/tpc.108.064360
PubMed Abstract | CrossRef Full Text | Google Scholar
Lin, T. P., Caspar, T., Somerville, C., and Preiss, J. (1988a). A starch deficient mutant of Arabidopsis thaliana with low ADPglucose pyrophosphorylase activity lacks i of the two subunits of the enzyme. Constitute Physiol. 88, 1175–1181. doi: x.1104/pp.88.iv.1175
PubMed Abstract | CrossRef Full Text | Google Scholar
Lin, T. P., Caspar, T., Somerville, C., and Preiss, J. (1988b). Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) heynh. lacking ADPglucose pyrophosphorylase activity. Plant Physiol. 86, 1131–1135. doi: 10.1104/pp.86.four.1131
PubMed Abstract | CrossRef Full Text | Google Scholar
López-Calcagno, P. E., Amani, O. A., Lawson, T., and Raines, C. A. (2017). Arabidopsis CP12 mutants take reduced levels of phosphoribulokinase and impaired office of the Calvin–Benson bike. J. Exp. Bot. 68, 2285–2298. doi: x.1093/jxb/erx084
PubMed Abstract | CrossRef Full Text | Google Scholar
Lu, Y., Gehan, J. P., and Sharkey, T. D. (2005). Daylength and cyclic effects on starch degradation and maltose metabolism. Plant Physiol. 138, 2280–2291. doi: 10.1104/pp.105.061903
PubMed Abstruse | CrossRef Full Text | Google Scholar
MacNeill, Grand. J., Mehrpouyan, S., Minow, M. A. A., Patterson, J. A., Tetlow, I. J., Emes, M. J., et al. (2017). Starch as a source, starch as a sink: the bifunctional role of starch in carbon resource allotment. J. Exp. Bot. 68, 4433–4453. doi: x.1093/jxb/erx291
PubMed Abstruse | CrossRef Full Text | Google Scholar
Marri, L., Trost, P., Trivelli, X., Gonnelli, L., Pupillo, P., and Sparla, F. (2008). Spontaneous assembly of photosynthetic supramolecular complexes every bit mediated by the intrinsically unstructured protein CP12. J. Biol. Chem. 283, 1831–1838. doi: 10.1074/jbc.M705650200
PubMed Abstract | CrossRef Total Text | Google Scholar
Marri, L., Zaffagnini, M., Collin, V., Issakidis-Bourguet, E., Lemaire, South. D., Pupillo, P., et al. (2009). Prompt and piece of cake activation by specific thioredoxins of Calvin bicycle enzymes of Arabidopsis thaliana associated in the GAPDH/CP12/PRK supramolecular complex. Mol. Plant ii, 259–269. doi: x.1093/mp/ssn061
PubMed Abstract | CrossRef Total Text | Google Scholar
Meyer, Y., Riondet, C., Constans, L., Abdelgawwad, G. R., Reichheld, J. P., and Vignols, F. (2006). Development of redoxin genes in the green lineage. Photosynth. Res. 89, 179–192. doi: 10.1007/s11120-007-9273-y
PubMed Abstract | CrossRef Full Text | Google Scholar
Michalska, J., Zauber, H., Buchanan, B. B., Cejudo, F. J., and Geigenberger, P. (2009). NTRC links born thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc. Natl. Acad. Sci. UsA. 106, 9908–9913. doi: 10.1073/pnas.0903559106
PubMed Abstract | CrossRef Full Text | Google Scholar
Michelet, L., Zaffagnini, M., Morisse, S., Sparla, F., Pérez-Pérez, K. E., Francia, F., et al. (2013). Redox regulation of the Calvin-Benson cycle: something old, something new. Front end. Plant Sci. four:470. doi: 10.3389/fpls.2013.00470
PubMed Abstract | CrossRef Total Text | Google Scholar
Mikkelsen, R., Mutenda, K. E., Mant, A., Schürmann, P., and Blennow, A. (2005). Blastoff-glucan, water dikinase (GWD): a plastidic enzyme with redox-regulated and coordinated catalytic activity and binding analogousness. Proc. Natl. Acad. Sci. U.S.A. 102, 1785–1790. doi: 10.1073/pnas.0406674102
PubMed Abstract | CrossRef Full Text | Google Scholar
Naranjo, B., Diaz-Espejo, A., Lindahl, M., and Cejudo, F. J. (2016). Type-f thioredoxins have a role in the brusk-term activation of carbon metabolism and their loss affects growth under short-24-hour interval weather condition in Arabidopsis thaliana. J. Exp. Bot. 67, 1951–1964. doi: 10.1093/jxb/erw017
PubMed Abstract | CrossRef Full Text | Google Scholar
Niittylä, T., Messerli, One thousand., Trevisan, Chiliad., Chen, J., Smith, A. M., and Zeeman, S. C. (2004). A previously unknown maltose transporter essential for starch degradation in leaves. Science 303, 87–89. doi: 10.1126/science.1091811
PubMed Abstruse | CrossRef Full Text | Google Scholar
Nikkanen, L., Toivola, J., and Rintamäki, E. (2016). Crosstalk betwixt chloroplast thioredoxin systems in regulation of photosynthesis. Found Cell Environ. 39, 1691–1705. doi: 10.1111/pce.12718
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ojeda, 5., Pérez-Ruiz, J. M., González, M.-C., Nájera, V. A., Sahrawy, M., Serrato, A. J., et al. (2017). N-concluding thioredoxin reductase domains and thioredoxins deed concertedly in seedling development. Plant Physiol. 174, 1436–1448. doi: x.1104/pp.17.00481
PubMed Abstract | CrossRef Full Text | Google Scholar
Okegawa, Y., and Motohashi, M. (2015). Chloroplastic thioredoxin m functions as a major regulator of Calvin cycle enzymes during photosynthesis in vivo. Establish J. 84, 900–913. doi: 10.1111/tpj.13049
PubMed Abstruse | CrossRef Full Text | Google Scholar
Pérez-Pérez, Thou. Eastward., Mauriès, A., Maes, A., Tourasse, N. J., Hamon, M., Lemaire, S. D., et al. (2017). The deep thioredoxome in Chlamydomonas reinhardtii: new insights into redox regulation. Mol. Plant 10, 1107–1125. doi: 10.1016/j.molp.2017.07.009
PubMed Abstract | CrossRef Total Text | Google Scholar
Pérez-Ruiz, J. Chiliad., Naranjo, B., Ojeda, V., Guinea, M., and Cejudo, F. J. (2017). NTRC-dependent redox balance of two-Cys peroxiredoxins is needed for optimal function of the photosynthetic apparatus. Proc. Natl. Acad. Sci. U.Due south.A. 114, 12069–12074. doi: 10.1073/pnas.1706003114
PubMed Abstract | CrossRef Full Text | Google Scholar
Pirone, C., Gurrieri, L., Gaiba, I., Adamiano, A., Valle, F., Trost, P., et al. (2017). The analysis of the different functions of starch-phosphorylating enzymes during the development of Arabidopsis thaliana plants discloses an unexpected role for the cytosolic isoform GWD2. Physiol. Institute. 160, 447–457. doi: 10.1111/ppl.12564
PubMed Abstract | CrossRef Full Text | Google Scholar
Pozueta-Romero, J., Ardila, F., and Akazawa, T. (1991). ADP-glucose ship by the chloroplast adenylate is linked to starch biosynthesis. Institute Physiol. 97, 1565–1572. doi: 10.1104/pp.97.iv.1565
PubMed Abstract | CrossRef Full Text | Google Scholar
Prasch, C. M., Ott, K. Five., Bauer, H., Anguish, P., Hedrich, R., and Sonnewald, U. (2015). ß-amylase1 mutant Arabidopsis plants bear witness improved drought tolerance due to reduced starch breakdown in baby-sit cells. J. Exp. Bot. 66, 6059–6067. doi: 10.1093/jxb/erv323
PubMed Abstract | CrossRef Total Text | Google Scholar
Ritte, Grand., Lloyd, J. R., Eckermann, N., Rottmann, A., Kossmann, J., and Steup, M. (2002). The starch-related R1 protein is an α-glucan, h2o dikinase. Proc. Natl. Acad. Sci. U.S.A. 99, 7166–7171. doi: 10.1073/pnas.062053099
PubMed Abstruse | CrossRef Full Text | Google Scholar
Sanz-Barrio, R., Corral-Martinez, P., Ancin, M., Segui-Simarro, J. M., and Farran, I. (2013). Overexpression of plastidial thioredoxin f leads to enhanced starch accumulation in tobacco leaves. Plant Biotechnol. J. 11, 618–627. doi: x.1111/pbi.12052
PubMed Abstract | CrossRef Full Text | Google Scholar
Seung, D., Thalmann, 1000., Sparla, F., Abou Hachem, Chiliad., Lee, S. K., Issakidis-Bourguet, Eastward., et al. (2013). Arabidopsis thaliana AMY3 is a unique redox-regulated chloroplastic α-amylase. J. Biol. Chem. 288, 33620–33633. doi: 10.1074/jbc.M113.514794
PubMed Abstract | CrossRef Full Text | Google Scholar
Silver, D. Grand., Silva, 50. P., Issakidis-Bourguet, E., Glaring, K. A., Schriemer, D. C., and Moorhead, G. B. G. (2013). Insight into the redox regulation of the phosphoglucan phosphatase SEX4 involved in starch degradation. FEBS J. 280, 538–548. doi: ten.1111/j.1742-4658.2012.08546.10
PubMed Abstruse | CrossRef Full Text | Google Scholar
Skeffington, A. W., Graf, A., Duxbury, Z., Gruissem, Westward., and Smith, A. M. (2014). Glucan, water dikinase exerts little control over starch degradation in Arabidopsis leaves at night. Institute Physiol. 165, 866–879. doi: 10.1104/pp.114.237016
PubMed Abstract | CrossRef Total Text | Google Scholar
Skryhan, Thousand., Cuesta-Seijo, J. A., Nielsen, M. K., Marri, L., Mellor, S. B., Glaring, Yard. A., et al. (2015). The role of cysteine residues in redox regulation and protein stability of Arabidopsis thaliana starch synthase i. PLoS Ane x:e0136997. doi: 10.1371/journal.pone.0136997
PubMed Abstruse | CrossRef Total Text | Google Scholar
Sparla, F., Costa, A., Schiavo, F., Lo Pupillo, P., and Trost, P. (2006). Redox regulation of a novel plastid-targeted b -amylase. Plant Physiol. 141, 840–850. doi: x.1104/pp.106.079186
PubMed Abstract | CrossRef Full Text | Google Scholar
Stitt, Yard., and Heldt, H. W. (1981). Simultaneous synthesis and degradation of starch in spinach chloroplasts in the light. Biochim. Biophys. Acta Bioenerg. 638, one–11. doi: x.1016/0005-2728(81)90179-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Thalmann, M. R., Pazmino, D., Seung, D., Horrer, D., Nigro, A., Meier, T., et al. (2016). Regulation of leaf starch deposition by abscisic acid is of import for osmotic stress tolerance in plants. Plant Cell 28, 1860–1878. doi: 10.1105/tpc.sixteen.00143
PubMed Abstract | CrossRef Total Text | Google Scholar
Thormählen, I., Ruber, J., von Roepenack-Lahaye, E., Ehrlich, S.-M., Massot, Five., Hümmer, C., et al. (2013). Inactivation of thioredoxin f1 leads to decreased light activation of ADP-glucose pyrophosphorylase and altered diurnal starch turnover in leaves of Arabidopsis plants. Found. Cell Environ. 36, 16–29. doi: 10.1111/j.1365-3040.2012.02549.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Toivola, J., Nikkanen, L., Dahlström, Grand. M., Salminen, T. A., Lepistö, A., Vignols, H. F., et al. (2013). Overexpression of chloroplast NADPH-dependent thioredoxin reductase in Arabidopsis enhances leafage growth and elucidates in vivo part of reductase and thioredoxin domains. Front. Found Sci. iv:389. doi: ten.3389/fpls.2013.00389
PubMed Abstruse | CrossRef Full Text | Google Scholar
Valerio, C., Costa, A., Marri, L., Issakidis-Bourguet, Eastward., Pupillo, P., Trost, P., et al. (2011). Thioredoxin-regulated beta-amylase (BAM1) triggers diurnal starch degradation in guard cells, and in mesophyll cells under osmotic stress. J. Exp. Bot. 62, 545–555. doi: 10.1093/jxb/erq288
PubMed Abstract | CrossRef Total Text | Google Scholar
van Wijk, One thousand. J. (2015). Protein maturation and proteolysis in plant plastids, mitochondria, and peroxisomes. Annu. Rev. Found Biol. 66, 75–111. doi: 10.1146/annurev-arplant-043014-115547
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, S.-Chiliad., Chu, B., Lue, Due west.-Fifty., Yu, T.-S., Eimert, Thou., and Chen, J. (1997). adg2-1 represents a missense mutation in the ADPG pyrophosphorylase large subunit cistron of Arabidopsis thaliana. Establish J. 11, 1121–1126. doi: ten.1046/j.1365-313X.1997.11051121.x
PubMed Abstruse | CrossRef Full Text | Google Scholar
Wang, Due south.-Chiliad., Lue, West.-50., Yu, T.-South., Long, J.-H., Wang, C.-Northward., Eimert, K., et al. (1998). Characterization of ADG1, an Arabidopsis locus encoding for ADPG pyrophosphorylase minor subunit, demonstrates that the presence of the small subunit is required for large subunit stability. Plant J. 13, 63–70. doi: x.1046/j.1365-313X.1998.00009.ten
PubMed Abstract | CrossRef Full Text | Google Scholar
Weise, Southward. E., Schrader, Due south. One thousand., Kleinbeck, One thousand. R., and Sharkey, T. D. (2006). Carbon balance and cyclic regulation of hydrolytic and phosphorolytic breakup of transitory starch. Plant Physiol. 141, 879–886. doi: x.1104/pp.106.081174
PubMed Abstract | CrossRef Total Text | Google Scholar
Yoshida, One thousand., and Hisabori, T. (2017). Distinct electron transfer from ferredoxin–thioredoxin reductase to multiple thioredoxin isoforms in chloroplasts. Biochem. J. 474, 1347–1360. doi: 10.1042/BCJ20161089
PubMed Abstract | CrossRef Total Text | Google Scholar
Yoshida, Grand., Matsuoka, Y., Hara, Due south., Konno, H., and Hisabori, T. (2014). Singled-out redox behaviors of chloroplast thiol enzymes and their relationships with photosynthetic electron transport in Arabidopsis thaliana. Plant Cell Physiol. 55, 1415–1425. doi: 10.1093/pcp/pcu066
PubMed Abstract | CrossRef Total Text | Google Scholar
Yu, T. South., Kofler, H., Häusler, R. Eastward., Hille, D., Flügge, U. I., Zeeman, S. C., et al. (2001). The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch deposition in plants, and non in the chloroplast hexose transporter. Constitute Prison cell 13, 1907–1918.
PubMed Abstract | Google Scholar
Zanella, M., Borghi, G. L., Pirone, C., Thalmann, M., Pazmino, D., Costa, A., et al. (2016). β-amylase 1 (BAM1) degrades transitory starch to sustain proline biosynthesis during drought stress. J. Exp. Bot. 67, 1819–1826. doi: x.1093/jxb/erv572
PubMed Abstract | CrossRef Full Text | Google Scholar
Zeeman, S. C., Kossmann, J., and Smith, A. M. (2010). Starch: its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 61, 209–234. doi: 10.1146/annurev-arplant-042809-112301
PubMed Abstruse | CrossRef Full Text | Google Scholar
beaurepairewervelf.blogspot.com
Source: https://www.frontiersin.org/articles/10.3389/fpls.2018.01344/full
0 Response to "under what environmental conditions would a plant use its stored starch to power its metabolism"
Post a Comment